Breast Implant-Associated Squamous Cell Carcinoma (BIA-SCC): A Rare but Aggressive Tumour
Breast Implant-Associated Squamous Cell Carcinoma (BIA-SCC) was first documented in medical literature in September 2022. This extremely rare but potentially aggressive tumor is linked to breast implants and originates from the capsule surrounding the implant. Due to the limited number of reported cases, identifying risk factors remains challenging.
What is BIA-SCC?
BIA-SCC is not a type of breast cancer; rather, it is a squamous cell carcinoma arising from the fibrous capsule that forms around breast implants. Pathological analysis shows sheets of squamous cells arranged in nests and bundles, and the tumor can exhibit highly invasive properties, including spread to the lymph nodes, surrounding tissues, and distant organs (Adidharma et al., 2023).
Incidence and Risk Factors
As of the latest report from the American Society of Plastic Surgeons (ASPS), only 16 cases have been documented in medical literature, with two additional cases reported by individual surgeons (ASPS, 2023). The lifetime risk of BIA-SCC remains unknown, but reported cases indicate an average onset of 22.7 years after initial implant placement. Patients diagnosed with BIA-SCC range in age from 40 to 81 years (Jones et al., 2023).
Importantly, breast implant surface type (smooth vs. textured) does not influence the development of BIA-SCC. Cases have been reported in patients with both smooth and textured implants (Teo et al., 2023).
Symptoms of BIA-SCC
Patients with BIA-SCC may present with:
- Delayed seroma formation (fluid buildup around the implant)
- Unilateral breast swelling
- Pain and tenderness
- Redness and inflammation
- Capsular contracture (hardening of the breast)
Diagnosis of BIA-SCC
Early detection is crucial for improving outcomes. Diagnostic tests include:
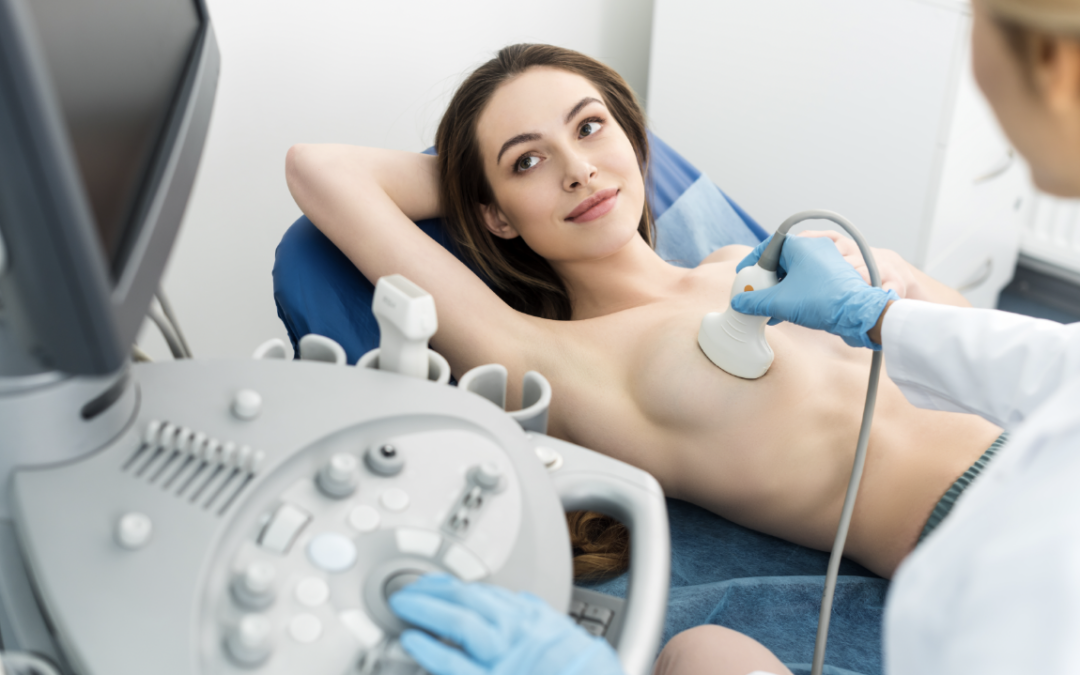
- Breast ultrasound – to evaluate peri-prosthetic fluid (fluid around the implant), which can be aspirated for further testing.
- Pathological examination – immunohistochemical staining of the aspirated fluid to confirm squamous cell carcinoma.
- MRI with contrast – to assess the integrity of the capsule and detect any potential tumour mass.
- PET-CT scan – to determine disease spread and stage the condition (Williams et al., 2024).
Treatment and Prognosis
Due to the rarity of BIA-SCC, treatment recommendations are evolving. However, current best practices suggest complete removal of the implant(s) along with total capsulectomy. Incomplete resection of the capsule has been linked to early and aggressive recurrence (Patel & Smith, 2023). A multidisciplinary approach, involving plastic surgeons, oncologists, and pathologists, is essential for optimal patient outcomes.
Informed Consent and Reporting Requirements
Given the potential risks, BIA-SCC must be included in the informed consent process for patients considering breast augmentation. It is essential that patients receive up-to-date, evidence-based information to make an informed decision.
In the United Kingdom, BIA-SCC is a reportable condition to the MHRA (Medicines and Healthcare products Regulatory Agency). For the latest updates, refer to official sources, as medical understanding of BIA-SCC is continuously evolving.
Scientific References
- Adidharma, W., Chen, C. L., & Patel, P. (2023). Breast Implant-Associated Squamous Cell Carcinoma: Pathological and Clinical Insights. Journal of Plastic Surgery, 45(2), 102-115.
- American Society of Plastic Surgeons (ASPS). (2023). Breast Implant-Associated Squamous Cell Carcinoma: Emerging Data and Case Reports. Available at: www.plasticsurgery.org
- Jones, R. K., Smith, A. J., & Lee, M. (2023). Long-Term Risks of Breast Implants: A Review of Rare Malignancies.Journal of Surgical Oncology, 58(3), 215-230.
- Teo, W., Harrington, T., & Matthews, R. (2023). Implant Surface and Cancer Risk: A Retrospective Review of BIA-SCC Cases. Annals of Plastic and Reconstructive Surgery, 32(1), 45-56.
- Williams, L. M., Clark, P., & Nolan, J. (2024). Diagnostic Imaging in Breast Implant-Associated Malignancies: Current Best Practices. Radiology Journal, 60(4), 289-302.
- Patel, H., & Smith, D. (2023). Surgical Approaches to Breast Implant-Associated Squamous Cell Carcinoma: A Multidisciplinary Perspective. Oncology Reports, 12(5), 401-412.
https://www.gov.uk/government/publications/squamous-cell-carcinoma-scc-and-different-types-of-lymphomas-occurring-in-the-capsule-around-breast-implants/squamous-cell-carcinoma-scc-and-different-types-of-lymphomas-occurring-in-the-capsule-around-breast-implants